Eye drop recall
Web 17 hours agoUS. The FDA warned consumers not to buy or use EzriCare Artificial Tears Delsam Pharmas Artificial Tears and Delsam.
9qh5htchxgadam
Web 20 hours agoEzriCare eye drops had been recalled back in February after being linked to 55 bacterial cases in 12 states.
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/CO2MZ3G7D5C3VMS5VYRQD7MTIQ.jpg)
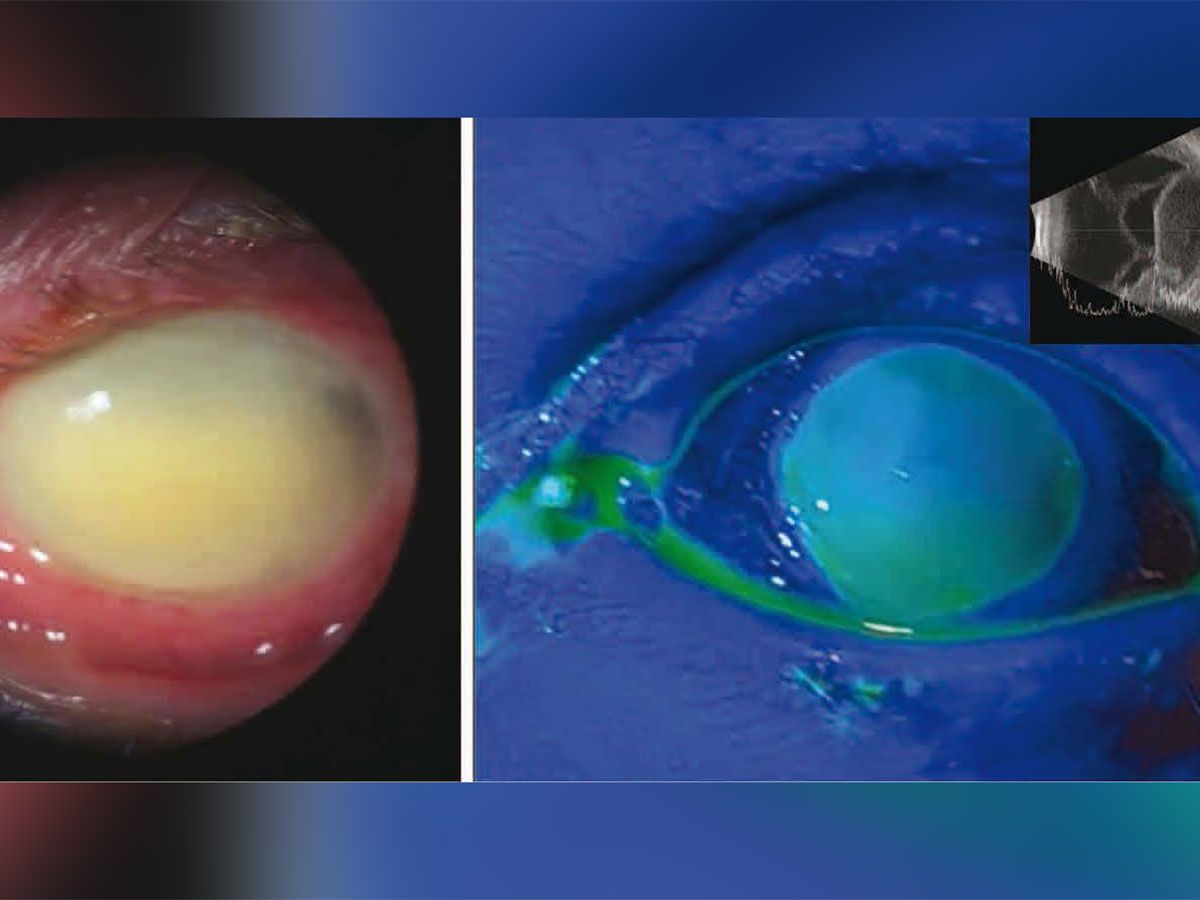
. Web 22 hours agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected. Web The eye drops were recalled in early February after the Centers for Disease Control and Prevention linked them to an outbreak of Pseudomonas aeruginosa. Officials are reporting two more deaths and additional cases of vision loss linked to eyedrops tainted with a drug-resistant bacteria.
Web 18 hours agoWhich eye drop brands have been recalled. Web Starting on July 3 2019 Altaire Pharmaceuticals began announcing a series of recall notices for a larger number of OTC and Rx eye drops gels and ointments. Web Of those who were able to recall brand names 85 said theyd used preservative-free EzriCare Artificial Tears Walters said.
CNN Global Pharma Healthcare is issuing a recall. Web According to the FDA the recalled eye drops were packaged in bottles with safety seals and small cartons with Ezricare drops having the NDC number 79503-0101. Web At the time of the recall there were 55 reports of adverse reactions to the drops including eye infections permanent vision loss and one death from a.
Web 2 hours agoPharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile. Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and. Web The Food and Drug Administration posted separate recall notices for certain eyedrops distributed by Pharmedica and Apotex after the companies said they are.
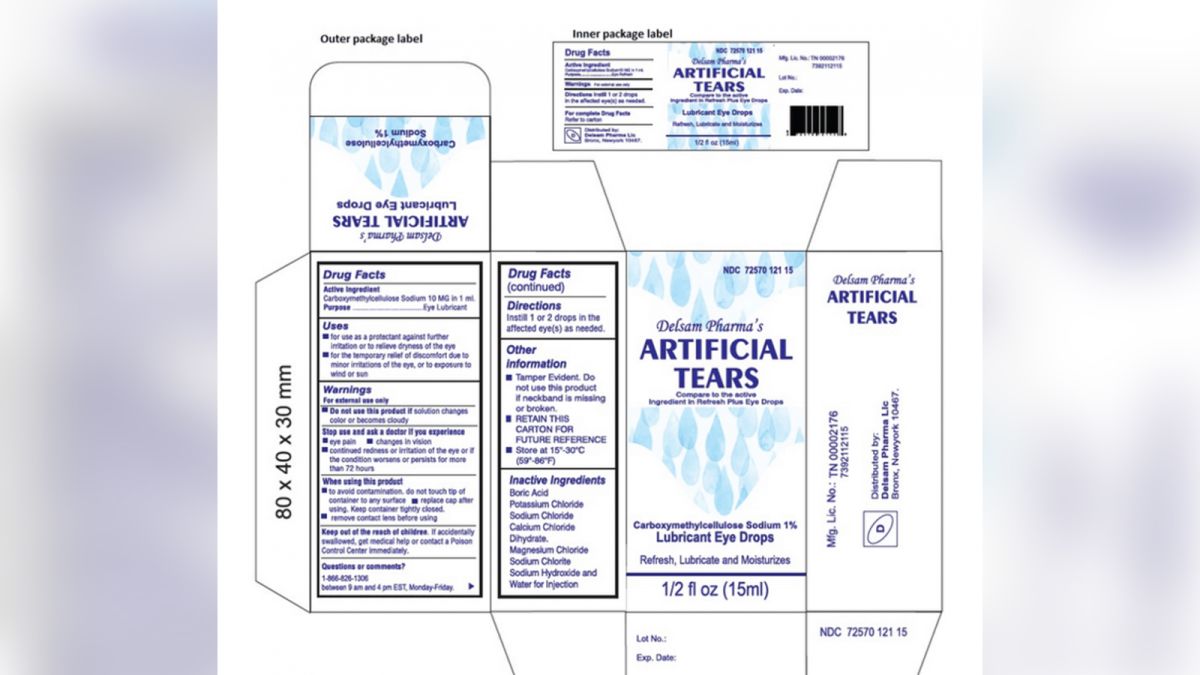
The drops have not been linked to illness the. Delsam Pharma eye drops are one of the brands subject to recall. Thirty-five patients were connected to four healthcare.
The CDC first alerted the public. Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from. The company recalled six lots.
Pharmedica is recalling its Purely Soothing 15 MSM Drops. Web Updated 815 PM ET Thu February 2 2023. Web On March 1 Apotex recalled prescription eye drops used to reduce eye pressure in people with glaucoma or ocular hypertension.
Web Getty Images. Web Most cases linked to the outbreak involved eye drops purchased online before the recall. However one reported buying EzriCare at a Costco warehouse.
Web 21 hours agoA rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one. Web WASHINGTON US. Web The FDA and CDC are advising patients to stop using EzriCare Artificial Tears an over-the-counter brand of eye drops.
Search for eye drops recall. Web A rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one. Browse common symptoms the most effective treatments.
Ad Find eye drops recall - symptoms causes conditions diagnosis treatment. Web Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops distributed by EzriCare LLC- and Delsam Pharma to the. Web Pharmedica USA issued a voluntary worldwide recall of two lots of its Purely Soothing 15 MSM eye drops the Food and Drug Administration announced March 3.
Web The companies involved in the recalls are Phoenix-based Pharmedica and Florida-based Apotex. A recall has been issued for a brand of eye drops and ointments sold at Walmart and Walgreens which the federal government says is not. The manufacturer Global Pharma.
Eye Drop Manufacturer Issues Recall Amid Cdc Investigation Of Infections Death Cnn
Several Eye Drops And Ointment Sold At Walmart Recalled For Being Potentially Non Sterile Wthr Com
Eye Drop Recall 2023 Here S What You Need To Know About The Flurry Of Eye Drop Recalls Cbs News

More Eye Drops Recalled This Time Over Infection Concerns From Non Sterility Marketwatch

Fda Eye Drops Recall Lawyer Contaminated Artificial Tears Attorney Hastings Law Firm Medical Malpractice Lawyers

Sipl92b1ica Am
:max_bytes(150000):strip_icc()/ezricare-e226fc86d3ab4331a7863d5ccee0e584.png)
Recalled Eye Drops Linked To Infections Vision Loss
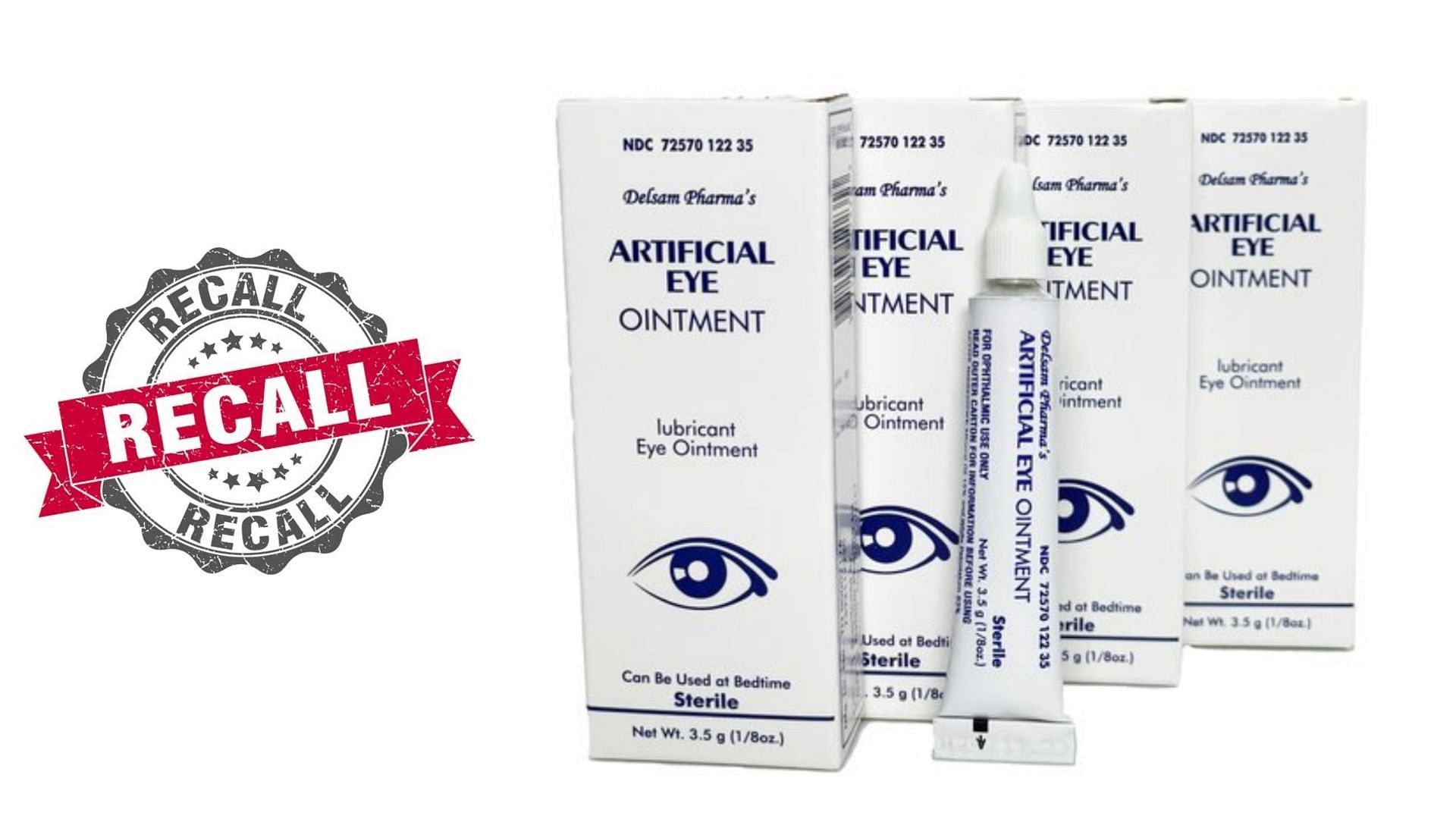
What Eye Drops Are Contaminated Fda Expands Warning Recall List
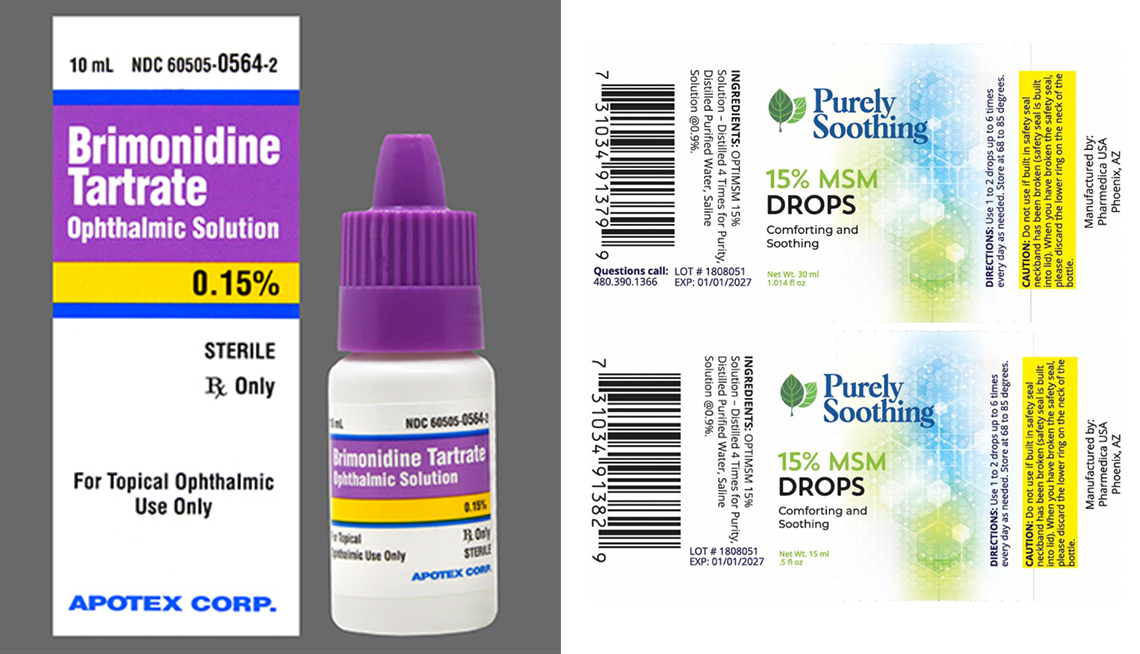
2 More Eye Drop Brands Recalled Due To Safety Risks
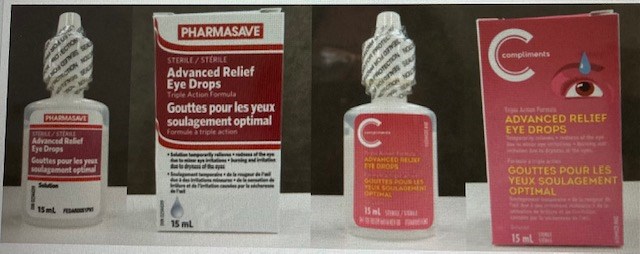
Pharmasave Advanced Relief Eye Drops Recalled After Packaging Error Poses Health Risks Nwonewswatch Com

Eyedrops Linked To Vision Loss Infections Have Been Recalled

Cdc Posts Update To Investigation Into Infections Linked To Eye Drops Cnet
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/7RXMEOM35FFEFBAMLZK7HWIWUM.jpg)
Recall Alert Fda Announces Two More Eyedrop Recalls Due To Potential Contamination Kiro 7 News Seattle
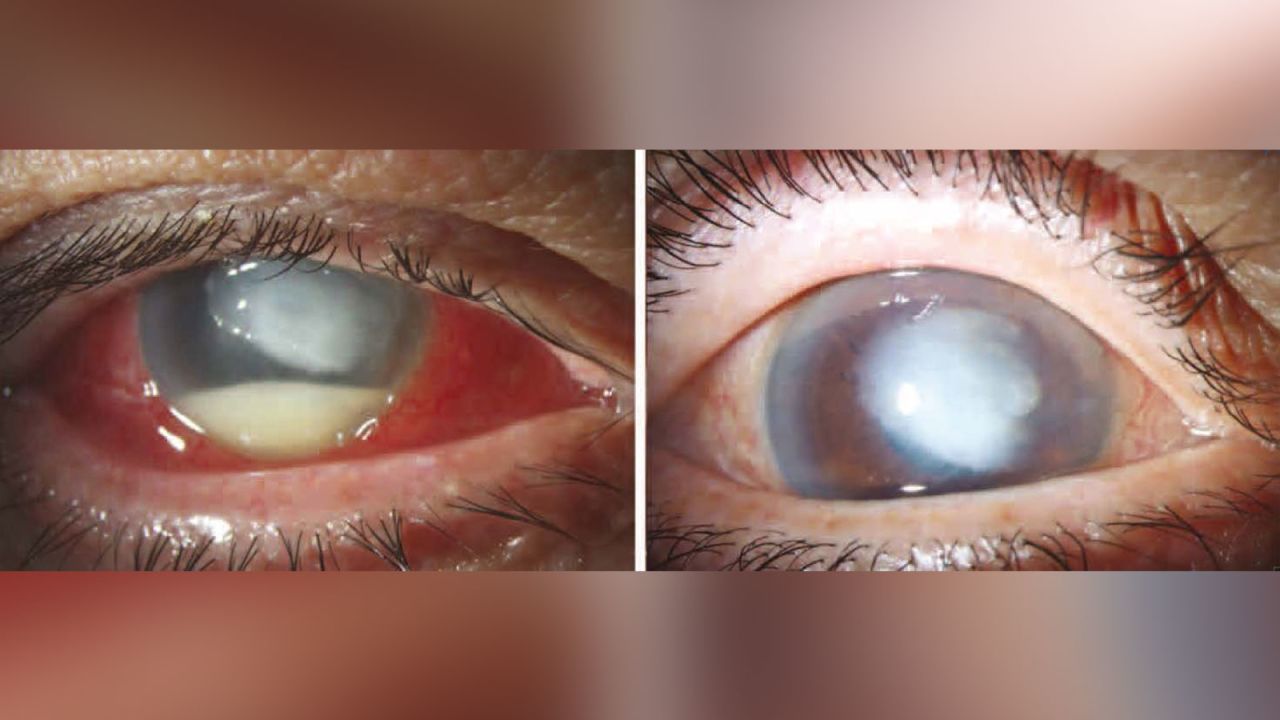
9qh5htchxgadam
Eye Drop Recall Announced Due To Possible Bacteria Contamination Top Class Actions

Eye Drops Sold Worldwide Recalled Due To Non Sterility Cnet

Several Eye Drops Ointment Sold At Walgreens Walmart Recalled Ktve Myarklamiss Com
Komentar
Posting Komentar